CSIRO begins testing Covid-19 vaccines
April 2nd, 2020: CSIRO
CSIRO, Australia’s national science agency, has commenced the first stage of testing potential vaccines for COVID-19. The testing, expected to take three months, is underway at CSIRO’s high-containment biosecurity facility, the Australian Animal Health Laboratory (AAHL) in Geelong.
To prepare for disease outbreaks, last year CSIRO partnered with the Coalition for Epidemic Preparedness Innovations (CEPI), a global group that aims to derail epidemics by speeding up the development of vaccines.
 In January, CEPI engaged CSIRO to start working on the virus SARS CoV-2, which causes the disease COVID-19. In consultation with the World Health Organisation, CEPI has identified vaccine candidates from The University of Oxford (UK) and Inovio Pharmaceuticals Inc. (US) to undergo the first pre-clinical trials at CSIRO, with further candidates likely to follow.
In January, CEPI engaged CSIRO to start working on the virus SARS CoV-2, which causes the disease COVID-19. In consultation with the World Health Organisation, CEPI has identified vaccine candidates from The University of Oxford (UK) and Inovio Pharmaceuticals Inc. (US) to undergo the first pre-clinical trials at CSIRO, with further candidates likely to follow.
The latest milestone builds on CSIRO’s growing work to tackle COVID-19, which has included scaling up other potential vaccine candidates at its biologics production facility in Melbourne.
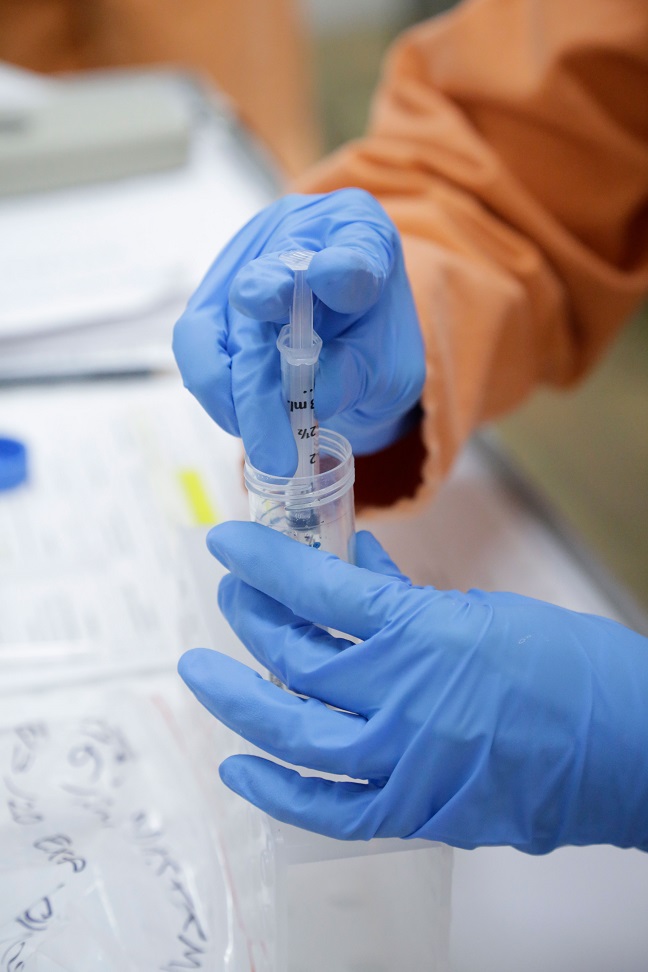
RIGHT: Scientists starting to test vaccines for COVID-19 at CSIRO's Australian Animal Health Laboratory. Credit: CSIRO.
CSIRO Chief Executive, Dr Larry Marshall said: “Beginning vaccine candidate testing at CSIRO is a critical milestone in the fight against COVID-19, made possible by collaboration both within Australia and across the globe.”
“CSIRO researchers are working around-the-clock to combat this disease which is affecting so many – whether it’s at the Australian Animal Health Laboratory (AAHL) or at our state-of-the-art biologics manufacturing facility – we will keep working until this viral enemy is defeated,” Dr Marshall said.
CSIRO is testing the COVID-19 vaccine candidates for efficacy, but also evaluating the best way to give the vaccine for better protection, including an intra-muscular injection and innovative approaches like a nasal spray.
Professor Trevor Drew OBE, is Director of AAHL and leading CSIRO’s COVID-19 virus and vaccine work.
“We have been studying SARS CoV-2 since January and getting ready to test the first vaccine candidates as soon as they are available," Professor Drew said.
"We are carefully balancing operating at speed with the critical need for safety in response to this global public health emergency.”
CSIRO has a long history of developing and testing vaccines since the opening of the AAHL in 1985. It is the only high biocontainment facility in the southern hemisphere working with highly dangerous and exotic pathogens, including diseases that transfer from animals to people.
Dr Marshall said: “Tackling disease and supporting better health outcomes takes a one-health approach."
“In 2016 CSIRO created the Health and Biosecurity research group who work with our scientists at AAHL to tackle our national and international health and biosecurity challenges together, so we can better protect the health of our people, environment, agriculture and industries and our way of life," Dr Marshall said.
“This, combined with our data science and manufacturing capability in our biological production facility, means we were well prepared to help Australia in One Health with disease identification, prevention and management, to deliver the real world solutions that our nation expects from science.”
CSIRO’s COVID-19 research so far
Current rapid work on COVID-19 spans virus and vaccine:
CSIRO was the first research organisation outside of China to generate sufficient stock of the virus —using the virus strain isolated by the Doherty Institute — to enable pre-clinical studies and research on COVID-19.
CSIRO successfully established a biological model in February 2020, the first in the world to confirm ferrets react to SARS-CoV-2 (the virus that causes COVID-19). Researchers have quickly progressed to studying the course of infection in the animals – a crucial step in understanding if a vaccine will work.
CSIRO researchers confirmed, after studying SARS CoV-2’s genomic sequence that the virus is presently changing into a number of distinct ‘clusters’ and are now starting to look at how this may also impact on the development of a vaccine.
For more information on CSIRO’s COVID-19 work, visit: www.csiro.au/COVID-19
About CEPI
CEPI is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines to stop future epidemics. CEPI has reached over US$750 million of its $1 billion funding target.
CEPI’s priority diseases include Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever and Chikungunya virus. CEPI also invests in platform technologies that can be used for rapid vaccine and immunoprophylactic development against unknown pathogens (ie, Disease X). To date, CEPI has committed to investing over $456 million in vaccine and platform development.
Nation's disease response bolstered
April 4, 2020
$220M directed to support expanded animal and human health remit Facility renamed Australian Centre for Disease Preparedness.
With the increasing threat of diseases spreading from animals to humans, including COVID-19, $220m will be directed to upgrading the high containment biosecurity research facility in Geelong, currently testing vaccines to combat the disease.
Operated by the CSIRO, Australia's national science agency, on behalf of the Australian Government, the facility's rapid work on COVID-19 has also been supported by a further $10 million in funding from the Government.
Formerly known as the Australian Animal Health Laboratory (AAHL), the research facility has been renamed the Australian Centre for Disease Preparedness (ACDP) to reflect its ongoing work to bring together human and animal health, disease protection and biosecurity measures, to better protect Australia to prevent, prepare for and control highly infectious diseases of animals as well as zoonotic diseases.
These diseases pass from animals to humans - such as COVID-19 and Severe Acute Respiratory Syndrome (SARS). Zoonotic diseases now account for almost 75 per cent of human infectious diseases.
CSIRO's research, spanning that undertaken at the ACDP, CSIRO's Health and Biosecurity research group, and CSIRO's state-of-the-art biologics manufacturing facility in Melbourne is forming an integral part of the rapid global response to the COVID-19 outbreak.
Dr Larry Marshall, Chief Executive of CSIRO said: "AAHL was originally created to protect Australia from animal diseases like foot and mouth, swine fever, and invasive species."
"But the emergence of Hendra virus in Australia demonstrated that diseases do not differentiate between animals and humans, so neither will we, as we step up our preparedness and response to both in a more holistic way.
"The centre will continue to build on the expertise delivered through AAHL's extensive biosecure laboratories combined with CSIRO's expertise across science disciplines to predict, prevent and manage disease, and turn the breakthroughs of Australia's medical research community into real world solutions for our greatest challenges, like pandemics."
The ACDP facility is unique to Australia and the southern hemisphere, forming a network of high biocontainment facilities worldwide able to enable work with highly dangerous and exotic pathogens.
Infections caused by these types of microbes are frequently fatal, affecting animals and people before the development of vaccines or treatments, such as with the disease COVID-19.
"Our scientists across CSIRO are working around the clock to address the battle against COVID-19 , but it is one we are well prepared for," Dr Marshall said.
"In 2016 we created our Health and Biosecurity business unit to work with our scientists at ACDP and we created our biological manufacturing facility.
"In partnership, and with many other research areas across CSIRO, we are tackling national and international health and biosecurity challenges together, so we can better protect the health of our people, environment, agriculture and industries, and our way of life."

Scientists working in the secure area at CSIRO's Australian Animal Health Laboratory (AAHL)
Opened in 1985, the ACDP is the only facility in Australia and the southern hemisphere capable of and licensed to carry out diagnosis and research on a range of such dangerous exotic pathogens.
CSIRO, with the ACDP at its core, has been at the forefront of work to combat viruses before. CSIRO developed the world's first effective flu treatment and a vaccine for the Hendra virus and, in collaboration with research partners, identified bats as the natural reservoir of Severe Acute Respiratory Syndrome (SARS)-like coronaviruses.
Professor Trevor Drew OBE, Director of the ACDP said: "The activity being undertaken by CSIRO at the ACDP is tripartite, reflecting a multisectoral, one-health approach to disease preparedness across the three sectors of humans, animals and the environment which they share."
"CSIRO is fully equipped and well prepared to deliver this capability on behalf of the nation, through its specialist facilities, its expert knowledge-base and its deep collaborative networks extending from academia to industry.
"The CSIRO COVID-19 work is an example of the criticality of a facility like ACDP. In providing a pipeline for rapid validation of a vaccine against this novel virus, we are carefully balancing operating at speed in response to a global public health emergency, we bridge the gap between academia and industry, in delivering the impactful and innovative science, for which CSIRO is renowned," Professor Drew said.

CSIRO's Australian Animal Health Laboratory in Geelong has been renamed the Australian Centre for Disease Preparedness (ACDP).